Wood Frog
Lithobates sylvaticus
Common Name: |
Wood Frog |
Scientific Name: |
Lithobates sylvaticus |
Etymology: |
|
Genus: |
Lithobates is Greek, Litho means "A stone", bates means "One that walks or haunts" |
Species: |
sylvaticus is Latin meaning "amidst the trees" |
Average Length: |
1.4 - 2.8 in. (3.5 - 7 cm) |
Virginia Record Length: |
|
Record length: |
3.3 in. (8.3 cm) |
Physical Description - This species ranges in length from 35-83 mm (1.5-3.25 in). It has a distinctive dark "mask" extending back from the eye. Dorsal coloration varies from nearly pink to shades of brown to nearly black. Females are typically more brightly colored and larger than the males. The venter of both sexes is white with a dark marking on both sides of the chest. Dorsolateral folds are prominent. The tadpole ranges in size from 42-48 mm and has no distinct markings on the body. The tail fin is rounded dorsally, tapers to a point and may be faintly patterned. The intestinal coil is partially visible. The oral disc is emarginated and has large papillae. The labial tooth ratio is 3/4 *1014* *11407*.
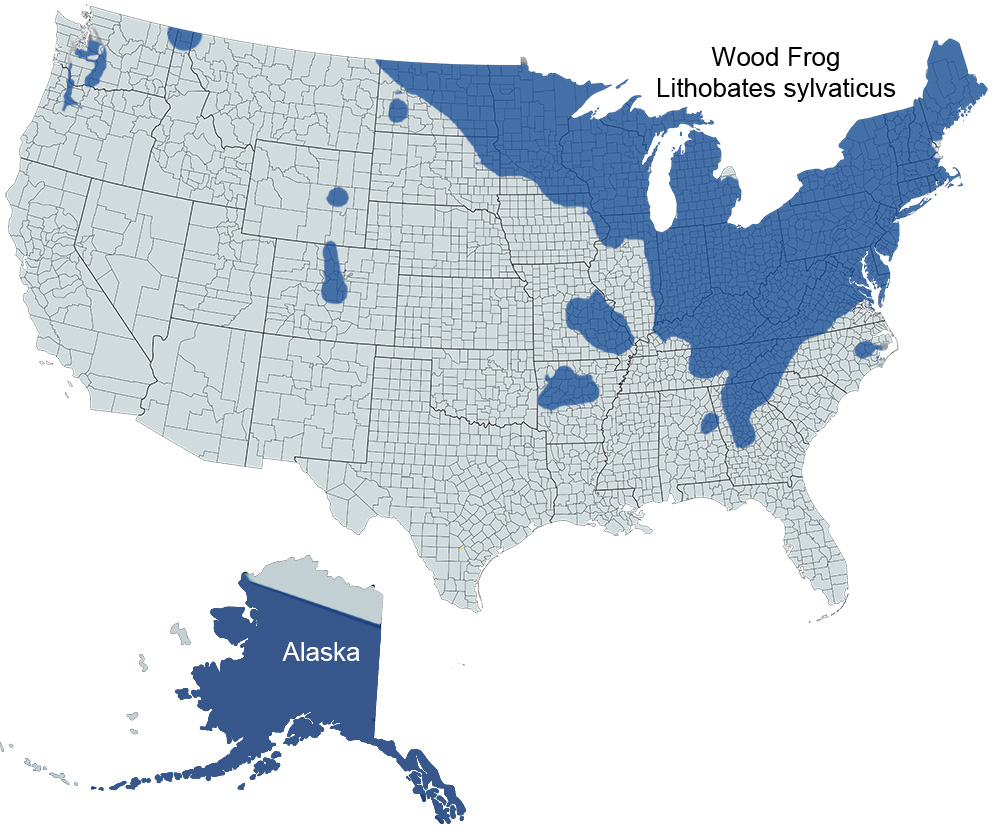
Historical Versus Current Distribution - Although their range is primarily boreal, Wood Frogs (Lithobates sylvaticus) are the most widespread North American amphibian species (Martof, 1970). They occur from the southern Appalachian Mountains of Georgia, north into Canada above the Arctic Circle, and west to Alaska (Martof, 1970; Conant and Collins, 1998). The western edge of their range runs roughly diagonally from Alaska and the Northwest Territories southeast through eastern North Dakota, the Upper Midwestern states (Minnesota, Wisconsin, and Illinois), and to northeastern Alabama. Disjunct populations occur in Colorado, Wyoming, and the Ozark Plateau (Martof, 1970; Conant and Collins, 1998), but reports of this species in Kansas are probably erroneous (Collins, 1993).
Wood Frogs have been the subject of at least two projects/studies of translocation as a conservation tool (Sexton and Phillips, 1986; Thurow, 1994). In both studies the preliminary results indicate at least short-term success, though the latter author has been criticized for translocating frogs from a donor area several hundred km away into an area for which historical presence of this species is not confirmed (Redmer, 1998b; Szafoni et al., 1999).
There have been no systematic studies done to compare current and historical distribution of Wood Frogs. No doubt populations have been lost as native landscapes have been anthropogenically altered, but it is likely that the current distribution resembles the historical distribution.
Historical versus Current Abundance - There are few quantified data against which historical and current abundance can be compared. Based on 11 yr of volunteer surveys of chorusing frogs, Mossman et al. (1998) reported a strong increase in numbers of Wood Frogs detected in Wisconsin, but noted that this was likely due to improvements in observer ability to detect them over the course of their study. Casper (1998) also noted that coordinated statewide Wisconsin atlas surveys conducted between 1981 and 1996 documented an increase of county records from 46 to 65 (41%). Fluctuations in adult populations and reproductive traits may be strongly influenced by larval fitness, mortality, metamorphosis, and recruitment (Berven, 1982a,b, 1988, 1990). Other studies have used museum specimens and recent studies to compare historical and current presence and absence (Davis et al., 1998; Pfingston, 1998; Redmer, 1998b).
Breeding - Reproduction is aquatic.
Breeding migrations - Breeding is well known to be "explosive" and occurs after the first warm rains of late winter or early spring (Banta, 1914; Wright and Wright, 1949; Howard, 1980; Seale, 1982b). Adults migrate from terrestrial overwintering sites to seasonal and semipermanent breeding wetlands. Timing of breeding season roughly follows latitudinal or elevational gradients. Examples (by state) of reported months in which breeding may occur include Alabama: January–February (Davis and Folkerts, 1979); Alaska: May–June (Herreid and Kinney, 1966, 1967); Arkansas: January–February (Trauth et al., 1989b, 1995a); Colorado and Wyoming: May (Corn and Livo, 1989); Georgia: February (Camp et al., 1990); Illinois (north): March–April (Pope, 1944); Illinois (south): February–March (Redmer, 2002); Maine: March–April (Hunter et al., 1999); Maryland: February (Berven, 1982a,b); Minnesota: March–April (Oldfield and Moriarty, 1994); Missouri: February (Guttman et al., 1991); New York: April (Wright, 1914; Wright and Wright, 1949); North Carolina: February–March (Hopey and Petranka, 1994); Pennsylvania: March–April (Seale, 1982b; Shaffer, 1991); Virginia: February (Berven, 1982a,b); Tennessee: February (Meeks and Nagel, 1973); Wisconsin: April–May (Mossman et al., 1998; Vogt, 1981).
Breeding habitat - Usually described as fish-free, ephemeral woodland pools or ponds or cut-off sections of streams within or adjacent to the adult habitat, though human-made aquatic habitats (including ditches, "wildlife" ponds, and tire ruts in dirt or clay roads) are commonly used as well (Trauth et al., 1989b, 1995a; Camp et al., 1990; Adams and Lacki, 1993; Hopey and Petranka, 1994; Cartwright et al., 1998; Trauth et al., 2000; Redmer, 2002). Assuming they are fish-free, colonization of small human-made pools such as tire ruts may benefit populations by increasing available breeding habitat, but such small habitats often have an insufficient hydroperiod to sustain eggs and tadpoles. Although the eggs can withstand some desiccation caused by temporary terrestrial standing (Forester and Lykens, 1988), tadpoles cannot. Eggs and tadpoles in small habitats may also be exposed to other risks including decreased food and increased exposure to predation and off-road vehicle traffic (Redmer, 2002). Presence of breeding ponds in open- versus closed-canopy ponds did not affect growth and survivorship (Werner and Glennemeier, 1999). Adults demonstrate considerable fidelity to certain breeding ponds, though a substantial number of juveniles disperse to breed in ponds other than their natal ponds (Berven and Grudzien, 1990). Recently, Paton et al. (2003) described breeding behavior during a drought.
Egg deposition sites - Oviposition sites have been characterized by many authors. Egg masses are usually deposited communally and are thus conspicuous. Communal oviposition is a possible function of explosive breeding, and additional studies have examined the relationships between mate choice, thermal advantage (freezing tolerance), and communal oviposition (Wells, 1977; Howard, 1980; Berven, 1981; Seale, 1982b; Waldman, 1982; Waldman and Ryan, 1983; Howard and Kluge, 1985; Corn and Livo, 1989; Trauth et al., 1995a; Cartwright et al., 1998). In particular, Waldman and Ryan (1983) point out a thermal advantage of up to 5 ˚C depending on position of egg, size of egg clump, and environmental conditions to communal egg laying in Wood Frogs. Egg masses are loose, and water convection between eggs is important for delivery of oxygen to developing embryos (Pinder and Friet, 1994).
Clutch size - Some older texts (Pope, 1944; Wright and Wright, 1949; Smith, 1961; Minton, 1972; Mount, 1975) cited a general range of 1,000–3,000 eggs/clutch, a figure probably based on imprecise estimates. However, some more-recent studies have consistently reported smaller numbers, from approximately 300–1,500 eggs/clutch (Berven, 1982a; Meeks and Nagel, 1973; Davis and Folkerts, 1986; Corn and Livo, 1989; Trauth et al., 1989b; Camp et al., 1990; Redmer, 2002).
Altig & McDiarmid 2015 - Classification and Description:
- Eastern Clump
- Arrangement 2 - Eggs oviposited as large clump, surface lobate even in old, melded clumps, structure is maintained until hatching.
- Sub-arrangement C - Eggs deposited in wooded nonflowing water; Ovum Diameter 1.8-2.4 mm; Egg Diameter 4.2-9.4 mm; 2 jelly layers; clump diameter 60-100 mm; clutch 2000-3000.
- Arrangement 2 - Eggs oviposited as large clump, surface lobate even in old, melded clumps, structure is maintained until hatching.
Length of larval stage - Tadpoles reach 50–60 mm TL at between 65–130 d (Wright and Wright, 1949; Herreid and Kinney, 1967; Meeks and Nagel, 1973; Berven, 1982b; Berven and Chadra, 1988; Camp et al., 1990; Redmer, 2002).
Tadpoles:
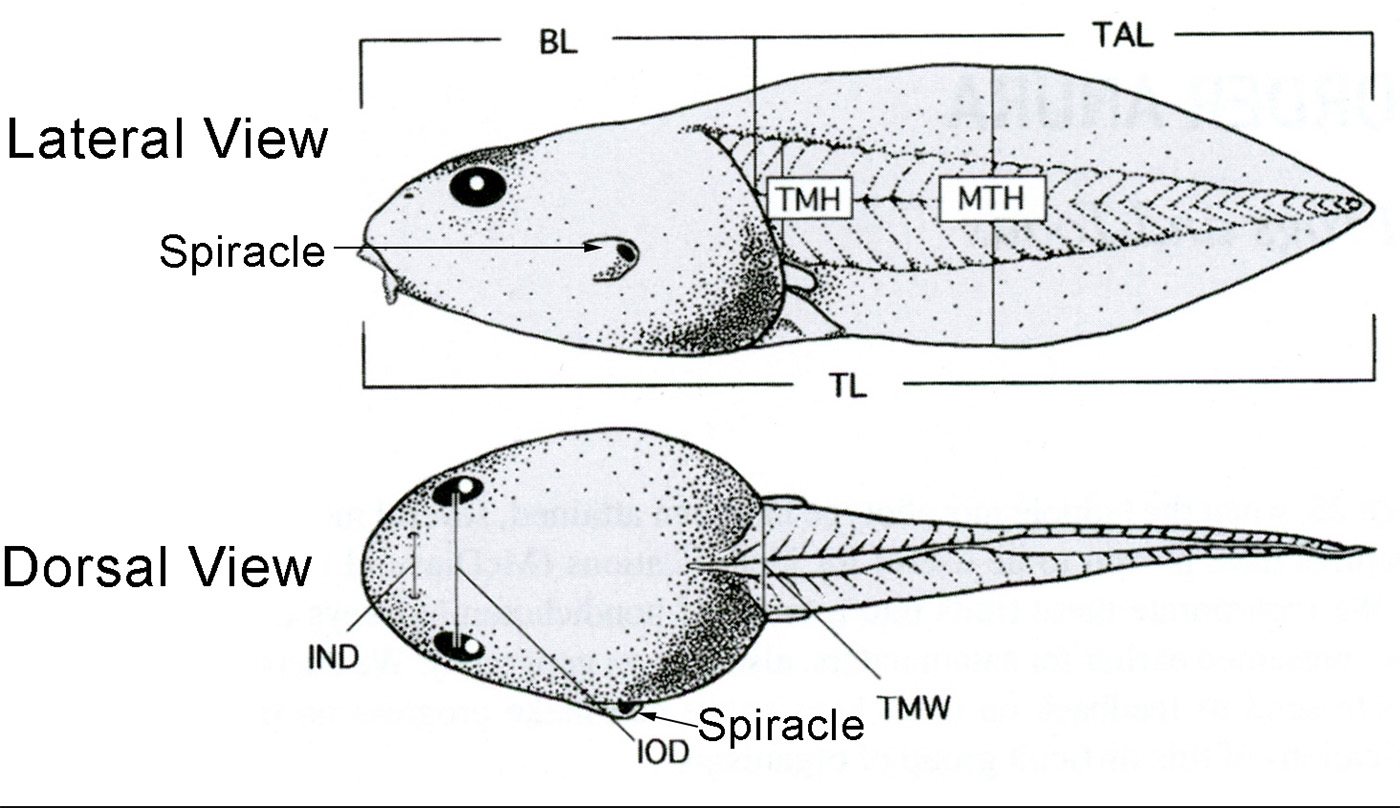
| Lateral View | Dorsal View |
|---|---|
| BL = Body Length | IND = Internarial Distance |
| MTH = Maximum Tail Height | IOD = Interorbital Distance |
| TAL = Tail Length | TMW = Tail Muscle Width |
| TL = Total Length | |
| TMH = Tail Muscle Height |
Larval requirements - Larvae feed opportunistically on detritus and plant material, but may also cannibalize conspecifics and prey upon other aquatic animals (Petranka et al., 1994; Petranka and Thomas, 1995; Petranka and Kennedy, 1999). Experimentally lengthened hydroperiods did not positively affect tadpole growth (Rowe and Dunson, 1995). Laboratory observations using spatial affinitiy as a recognition assay demonstrate sibling recognition (Fishwald et al., 1990).
Larval polymorphisms - Distinctive cannibal morphs are unknown, though cannibalism by large tadpoles upon eggs and smaller conspecific tadpoles is well known (Bleakney, 1958; Petranka and Thomas, 1995). Such predation increases with size of the cannibalistic tadpole (Petranka and Thomas, 1995). Phenotypic plasticity has been demonstrated experimentally in tadpoles raised in the presence of dragonfly larvae (Van Buskirk and Relyea, 1998; Relyea and Werner, 2000).
Features of metamorphosis - Metamorphosis occurs within 65–130 d (Wright and Wright, 1949; Herreid and Kinney, 1967; Meeks and Nagel, 1973; Berven, 1982b; Berven and Chadra, 1988; Camp et al., 1990; Redmer, 2002).
Post-metamorphic migrations - In a Virginia study of the effects of post-metamorphic dispersal on population genetic structure, up to 18% of juveniles were found to breed in ponds other than their own natal ponds (Berven and Grudzien, 1990).
Juvenile Habitat - Microhabitat preferences of juveniles are not specifically known, though observations of post-metamorphic juveniles indicate similarity to adults.
Adult Habitat - Wood Frogs are found in a variety of habitats including tundra, subalpine woodlands, willow thickets, wet meadows, bogs, and temperate forests (both coniferous and deciduous) of various canopy species associations (Martof and Humphries, 1959; Martof, 1970; Pentecost and Vogt, 1976; Vogt, 1981; Davis and Folkerts, 1986; Hammerson, 1986; Corn and Livo, 1989; DeGraaf and Rudis, 1990; Russell and Bauer, 1993; Oldfield and Moriarty, 1994; Trauth et al., 1995a; Harding, 1997; Behler and King, 1998; Conant and Collins, 1998; Redmer, 2002). Wood Frogs are sensitive to edge effects and reduced canopy cover created by forest cutting (deMaynadier and Hunter, 1998; Gibbs, 1998a,b). The relationship between landscape level distribution and population cycles was examined in southwest Ontario (Hecnar and McCloskey, 1996). Potential habitat has recently been described by Baldwin and Vasconcelos (2003).
Home Range Size - The only extensive study of this topic was a mark-recapture study conducted in Minnesota by Bellis (1961b), who found most recaptured adults near the initial capture site. The mean distance moved was 13.3 m2 (n = 298). Home range sizes were from 3.8–477.2 m2 (mean = 83.6 m2). Several studies examine movements (see "Seasonal Migrations" below). Though not specifically addressing home range size, one study of a Virginia population contributed extensive data on post-metamorphic dispersal and gene flow (Berven and Grudzien, 1990). In this study, up to 18% of juveniles dispersed to breed in ponds other than those of their origin, and maximum movement distances were reported to be 800–1,513 m. Cohorts formed mean genetic neighborhood areas of 5,035 ha, and gene flow between ponds was restricted beyond distances of 1,000 m (Berven and Grudzien, 1990).
Territories Unknown.
Aestivation/Avoiding Dessication - Unknown, but several authors provide evidence that in the warmer regions, cool or moist microhabitats are sought as temperature increases or humidity decreases (Heatwole, 1961a; Trauth et al., 1995a; Redmer, 2002).
Seasonal Migrations - Wood Frogs exhibit explosive, synchronous migrations to breeding ponds in late winter to early spring (Wright and Wright, 1949; Meeks and Nagel, 1973; Howard, 1980; Seale, 1982b). Movements within a forest habitat are restricted by road corridors (Gibbs, 1998a).
Torpor (Hibernation) - Wood Frogs hibernate terrestrially near the soil surface (Heatwole, 1961a; Bellis, 1962; Howard, 1980; Schmid, 1982; Storey, 1984; Storey and Storey, 1987; Zweifel, 1989; Licht, 1991). They are resistant to dehydration (Schmid, 1965b), but tend to hibernate nearer to breeding ponds; this tendency is more pronounced in males than females (Regosin et al., 2003).
Wood Frogs tolerate sub-freezing temperatures by producing cryoprotectants. The literature on this and related physiological responses is extensive (see Costanzo and Lee, 1994, for a review). Adults from southern populations have higher critical thermal maxima (CTM) then non-acclimated individuals from more northern populations, but there is no difference in tolerance to low temperatures (Lotshaw, 1977; Manis and Claussen, 1986).
Interspecific Associations/Exclusions - Although they are broadly sympatric, Wood Frogs and Northern Leopard Frogs (L. pipiens) are reported to infrequently breed in the same ponds, and while no single cause for this segregation has been determined, food, predation, and habitat segregation may all contribute (DeBenedictis, 1974; Werner and Glennemeier, 1999). Wood Frog tadpoles are known to prey upon eggs and tadpoles of American Toads (Anaxyrus americanus), and female American Toads avoid oviposition in ponds where Wood Frogs are present (Petranka et al., 1994). In turn, the larvae of several species of ambystomatid salamanders are important predators of tadpoles and egg masses of Wood Frogs (see “Predators,” below).
Age/Size at Reproductive Maturity - Males mature within 1–2 yr of metamorphosis, females usually at 2–3 yr (Berven, 1982a; Bastien and Leclair, 1992; Sagor et al., 1998; Redmer, 2002). Differential rates of maturity are a suggested cause of different sex ratios usually seen in breeding ponds (Berven, 1990). Sizes of mature individuals are extremely variable across their range (Martof and Humphries, 1959; Smith, 1961; Minton, 1972; Meeks and Nagel, 1973; Berven, 1982a; Davis and Folkerts, 1986; Trauth et al., 1989b, 1995a; Bastien and Leclair, 1992; Redmer, 2002). Geographic variation in growing season, elevation, and tadpole fitness (size at metamorphosis) are all known to affect life history and demographic traits such as age/size at which maturity is attained (Martof and Humphries, 1959; Berven, 1982a,b, 1988, 1990; Berven and Gill, 1983; Davis and Folkerts, 1986; Sagor et al., 1998).
Longevity - In Québec and southern Illinois, estimated ages of adults are 4 yr for males and 5 yr for females (Bastien and Leclair, 1992; Redmer, 2002). In Minnesota (Bellis, 1961a), Maryland, and Virginia (Berven, 1982a), and another Québec study (Sagor et al., 1998), maximum reported ages are 3 yr for males and 4 yr for females.
Bastien and Leclair (1992) suggested mean physiological longevity (MPL = mean age in years multiplied by the average number of frost-free days in the region) as an alternative to expressing longevity in years, and commented on possible variation in MPL of different Wood Frog populations. From their data, and other published data (Berven, 1982a), they calculated MPL for three populations (Québec, Maryland, and Virginia). They speculated that MPL was relatively constant in females but geographically more variable in males, and that earlier maturity curtailed the potential life span of males. However, MPL calculated for southern Illinois Wood Frogs was much longer than those that Bastien and Leclair (1992) calculated (Redmer, 2002).
Larvae Feeding Behavior - Tadpoles may prey heavily upon eggs and embryos of some salamanders (Petranka et al., 1998).
Predators - The larvae of several ambystomatid salamanders are important predators of tadpoles and egg masses of Wood Frogs (Wilbur, 1972; Walters, 1975; Rowe and Dunson, 1995). Leeches are also known predators of embryos and tadpoles (Cory and Manion, 1953). Adults are preyed upon by a number of terrestrial and aquatic predators.
Anti-Predator Mechanisms - Eggs are reportedly highly palatable to some aquatic predators (Walters, 1975). Although Wood Frogs exhibit a high degree of philopatry (Berven and Grudzien, 1990), newly created ponds are rapidly colonized, and females select fish-free ponds as oviposition sites (Hopey and Petranka, 1994). Experiments have shown that tadpoles raised in the presence of certain predators adopt alternate phenotypes or behaviors to avoid predation (VanBuskirk and Relyea, 1998; Relyea and Werner, 1999, 2000; Relyea, 2000; but see Anderson and Petranka, 2003). In laboratory trials, post-metamorphic Wood Frogs (along with Pickerel Frogs [L. palustris]) were found to survive attacks by short-tailed shrews (Blarina brevicauda) more often than American Bullfrogs (L. catesbeianus) or Green Frogs (L. clamitans; Formanowicz and Brodie, 1979).
Diseases - Infection by Aeromonas sp. bacteria has been reported (Nyman, 1986). Mortality sometimes occurs in breeding choruses when multiple males attempt to amplex single females, resulting in "mating balls" that may drown the female (Phillips and Wade, 1990).
Parasites - The extensive literature on Wood Frog parasites was reviewed by McAllister et al. (1995e). In Canada alone, studies date back nearly 100 yr (Stafford, 1905; McAllister et al., 1995e). In the United States, data are available from Alaska (Metcalf, 1923), Arkansas (McAllister et al., 1995e), Maine (Bouchard, 1951), Maryland (Walton, 1931), Massachusetts (Rankin, 1945), Michigan (Najarian, 1955; Muzzall and Peebles, 1991), New York (Harwood, 1930, 1932), North Carolina (Metcalf, 1923), Ohio (Metcalf, 1923; Odlaug, 1954), and Wisconsin (Williams and Taft, 1980).
Protozoan parasites were reviewed in McAllister et al. (1995e). Metazoans infecting Wood Frogs include adult and/or larval stages of at least 10 trematodes, 3 cestodes, 8 nematodes, 1 acanthocephalian, and 1 hirudinean (McAllister et al., 1995e). A brief summary of the parasites from Wood Frogs in the United States is presented below; a listing of Canadian genera of helminth parasites (along with their references) can be found in McAllister et al. (1995e).
Protozoan parasites of U.S. populations, including Opalina spp., were found in the rectum of Wood Frogs from Arkansas (McAllister et al., 1995e) and Ohio (Metcalf, 1923), and Cepedea spp. were found from specimens in Alaska and Michigan. Wood Frogs were found to host the myxosporean Myxidium serotinum in Arkansas (McAllister et al., 1995e). A previously unnamed eimerian (Eimeria fitchi) has been found in the feces of specimens from Arkansas (McAllister et al., 1995e).
Trematodes documented from Wood Frogs include the plagiorchid, Brachycoelium salamandrae (Rankin, 1938; Odlaug, 1954; McAllister et al., 1995e; Najarian, 1955). Unidentified trematode metacercariae were mentioned in specimens from Arkansas (McAllister et al., 1995e), Massachusetts, and Michigan (Rankin, 1945; Najarian, 1955; Muzzall and Peebles, 1991), while echinostome cysts were found in Michigan populations (Najarian, 1955). The genus Gorgoderina and gorgoderid cysts have been found in several regions (Rankin, 1945; Bouchard, 1951; Najarian, 1955; Waitz, 1961). Four species of Haematoloechus have been reported (Waitz, 1961; Catalano and White, 1977; Williams and Taft, 1980; Muzzall and Peebles, 1991).
Tetrathyridia of the cyclophyllidean tapeworm genus Mesocestoides reported from Arkansas (McAllister et al., 1995e) also are found in Wood Frogs. Rankin (1945) observed plerocercoid larvae in Massachusetts Wood Frog populations.
Nematode genera known from Wood Frogs include Abbreviata, Cosmocercoides, Megalobatrachonema, Oswaldocruzia, Rhabdias, and Spiroxys from a number of U.S. states and Canada (Walton, 1931; Harwood, 1932; Rankin, 1945; Baker, 1978a, 1987; Muzzall and Peebles, 1991; McAllister et al., 1995e).
Odlaug (1954) found a cystacanth of an unidentified acanthocephalan from a Wood Frog in Ohio, and McAllister et al. (1995e) reported a widely distributed glossiphoniid leech, Desserobdella (= Batrachobdella) picta, attached to a single Wood Frog from Arkansas.
Conservation - Throughout much of their range, Wood Frogs are a common and familiar species. Many authors have made general comments concerning regional abundance or rarity (Dickerson, 1906; Wright and Wright, 1949; Mount, 1975; Pentecost and Vogt, 1976; Vogt, 1981; DeGraaf and Rudis, 1983; Green and Pauley, 1987; Johnson, 1987; Oldfield and Moriarty, 1994; Harding, 1997; Hunter et al., 1999). However, in several states along the periphery of their range they are either considered by local experts to be Restricted, Uncommon, or Rare, or they are afforded legal protection. These include Alabama (Mount, 1975), Arkansas (Trauth et al., 1989b, 1995a), Colorado (Corn and Livo, 1989; Levell, 1997), Illinois (Ackerman, 1975; Mierzwa, 1998a; Redmer, 1998b), Missouri (Johnson, 1987), and New Jersey (Levell, 1997).
References for Life History
- Altig, Ronald & McDiarmid, Roy W. 2015. Handbook of Larval Amphibians of the United States and Canada. Cornell University Press, Ithaca, NY. 341 pages.
- AmphibiaWeb. 2020. University of California, Berkeley, CA, USA.
- Conant, Roger and, Collins, John T., 2016, Peterson Field Guide: Reptiles and Amphibians, Eastern and Central North America, 494 pgs., Houghton Mifflin Company., New York
- Duellman, William E. and, Trueb, Linda, 1986, Biology of Amphibians, 671 pgs., The Johns Hopkins University Press, Baltimore
- Martof, B.S., Palmer, W.M., Bailey, J.R., Harrison, III J.R., 1980, Amphibians and Reptiles of the Carolinas and Virginia, 264 pgs., UNC Press, Chapel Hill, NC
- Wilson, L.A., 1995, Land manager's guide to the amphibians and reptiles of the South, 360 pp. pgs., The Nature Conservancy, Southeastern Region, Chapel Hill, NC
Photos:
*Click on a thumbnail for a larger version.
Verified County/City Occurrence
Accomack
Albemarle
Alleghany
Amherst
Appomattox
Arlington
Augusta
Bath
Bedford
Bland
Botetourt
Buchanan
Buckingham
Campbell
Caroline
Clarke
Craig
Cumberland
Dickenson
Fairfax
Fauquier
Floyd
Fluvanna
Franklin
Frederick
Giles
Goochland
Grayson
Greene
Hanover
Henry
Highland
King and Queen
King George
Lee
Loudoun
Madison
Montgomery
Nelson
Page
Patrick
Pittsylvania
Powhatan
Prince Edward
Prince William
Pulaski
Roanoke
Rockbridge
Rockingham
Russell
Scott
Shenandoah
Smyth
Spotsylvania
Stafford
Warren
Washington
Westmoreland
Wise
CITIES
Alexandria
Buena Vista
Danville
Fairfax
Lynchburg
Verified in 59 counties and 5 cities.
U.S. Range