Cope's Gray Treefrog
Dryophytes chrysoscelis
Male Aggression Call
Common Name: |
Cope's Gray Treefrog |
Scientific Name: |
Dryophytes chrysoscelis |
Etymology: |
|
Genus: |
Dryophytes The name "Dryophytes" is derived from the Greek words "dryos," meaning tree, and "phytes," meaning plant, which refers to the arboreal habits of these frogs. |
Species: |
chrysoscelis is derived from the Greek words chryso which means 'gold' and kelis which means "a spot". This refers to the orange-yellowish spotting on the inner thigh. |
Average Length: |
1.3 - 2 in. (3.2 - 5.1 cm) |
Virginia Record Length: |
|
Record length: |
2.4 in. (6 cm) |
*Note: Our two native gray treefrogs are identical in appearance. In the field the only two ways to distinguish D. chrysoscelis from D. versicolor is by their call and in some cases geographic location.
Cope’s Gray Treefrogs (Dryophytes chrysoscelis) and Gray Treefrogs (Dryophytes versicolor) are members of a cryptic, diploid-tetraploid species complex. This has resulted in considerable taxonomic confusion, especially in early reports. As a result of this confusion, many authors have chosen to combine the accounts and distributions of these species. In this account, I have attempted to separate, as much as possible, the literature related to D. chrysoscelis and D. versicolor. For categories where data are lacking or where there is a question regarding the species identification, data from the sister species are reported. This approach is at least partially validated by the genetic analysis of this complex by Ptacek et al. (1994) and the growth and development studies of Ptacek (1996).
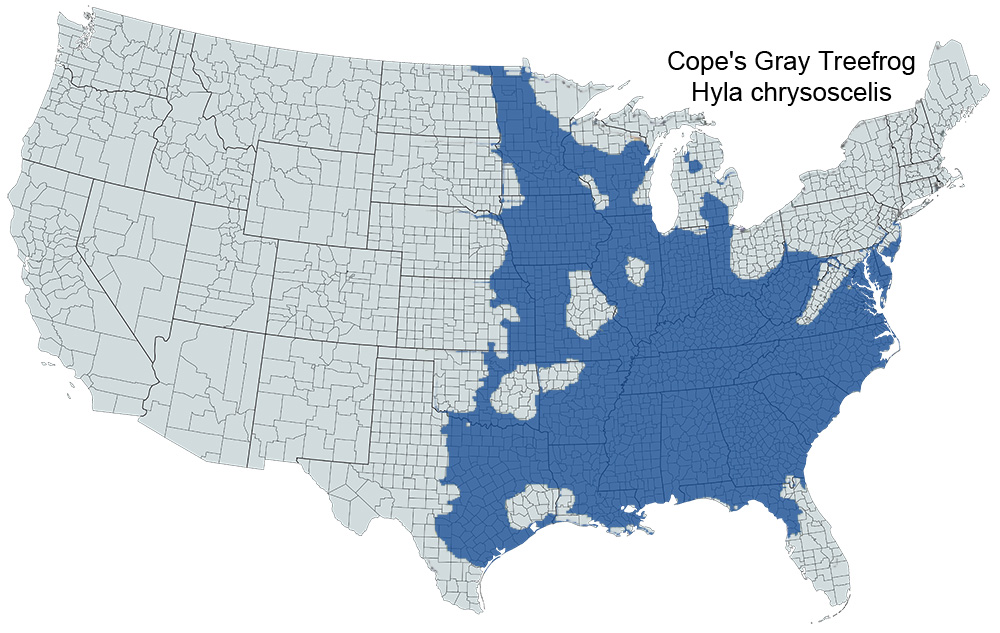
Historical versus Current Distribution - Because of the cryptic nature of Cope’s gray treefrogs and Gray Treefrogs, discussions of these species are nearly always intertwined. Cope's Gray Treefrogs were originally designated as a subspecies of pine woods treefrogs (Dryophytes femoralis chrysoscelis; Cope, 1880). Wright and Wright (1949) listed Cope's gray treefrogs as subspecies of Gray Treefrogs (Dryophytes versicolor chrysoscelis). The distribution of Cope's Gray Treefrogs has long been associated, and usually combined, with that of Gray Treefrogs. Noble and Hassler (1936) reported two call types along the East Coast of the United States. A fast-trilling, harsh call type was found in the southern United States, while a slower trilling, more mellow sounding call type was found at higher latitudes. The two call types coexisted in a railroad yard in Baltimore, Maryland. Wright and Wright (1949), apparently not realizing the importance of the call differences, restricted the distribution of D. v. chrysoscelis to east-central Texas, east into southern Arkansas and northwestern Louisiana. Blair (1958a) reported distinct geographic distributions for two call types of what was then considered D. versicolor. Recent depictions show the distribution of the gray treefrog complex to encompass much of the eastern United States, including the eastern Great Plains (eastern portions of Nebraska, Kansas, Oklahoma, and eastern Texas), Minnesota south to Louisiana, and east of the Mississippi River to the Atlantic coastline, excluding northern Maine and peninsular Florida, south of the panhandle (Conant and Collins, 1998).
Species identification has long been problematic in this group. Noble and Hassler (1936) first reported discrete call types along a latitudinal cline. Blair (1958a) plotted distinct geographic distributions of two call types across the range of what was then considered D. versicolor. Johnson (1959, 1961, 1963, 1966) reported genetic incompatibility between the call types, prompting him to designate the faster trilling call type as D. chrysoscelis and the slower trilling call type as D. versicolor. The diploid-tetraploid nature of this complex was first suggested when Wasserman (1970) reported that D. versicolor was a tetraploid (4N = 48). Later, Bogart and Wasserman (1972) confirmed the diploid (2N = 24) nature of D. chrysoscelis. Ralin (1977a) discussed call variation between D. chrysoscelis and D. versicolor along an east-west transect from central Texas to Louisiana. Gerhardt (1974a), Ralin (1977a), and Cline (1990) quantified call differences between eastern and western races of D. chrysoscelis. Bachmann and Bogart (1975), Cash and Bogart (1978), and Green (1980) demonstrated partial discrimination between the two species by measuring cell diameter. Ralin and Rogers (1979) reported that D. versicolor were morphologically and genetically (Ralin and Selander, 1979) intermediate between eastern and western populations of D. chrysoscelis. Cline (1990) combined acoustical, morphological, and genetic analyses in central Oklahoma, Kansas, Missouri, and Arkansas to show Cope's Gray Treefrogs range from east-central Texas (excluding the eastern 1/5 of the state), north into central Oklahoma (excluding the eastern 1/4 of the state), eastern Kansas, extreme eastern Nebraska, extreme eastern South Dakota, extreme eastern North Dakota, western and southern Minnesota, Iowa, extreme southwest Wisconsin, most of Missouri, east in a band across south-central Illinois, much of central Indiana, and much of western and central Ohio, south through most of Arkansas and Louisiana, Mississippi, southwestern Tennessee, Alabama, the Florida Panhandle, Georgia, South Carolina, eastern North Carolina, southeastern Virginia, and southeastern Maryland.
Numerous attempts have been made to identify and determine the distributions of these cryptic species at the state level. Hoffman (1946, Virginia), Walker (1946, Ohio), Brown and Brown (1972, Illinois), Bogart and Jaslow (1979, Michigan), Johnson (1987, Missouri), Matson (1988, Ohio), and Little (1983, Ohio/West Virginia) used call characteristics. Olson (1984) used calls and karyotypes to verify D. chrysoscelis in Minnesota. Jaslow and Vogt (1977, Wisconsin), Chapin and Trauth (1987, Arkansas), and Hillis et al. (1987, Kansas) used histological techniques. Recent research at the state level has begun to elucidate the separate distributions of Cope's and Gray Treefrogs.
Historical versus Current Abundance - Little data comparing historical and current abundance are available. With the advent of the North American Amphibian Monitoring Program (NAAMP) and its standardized protocols, this paucity of data should be resolved. Most of the early reports suggest, at least qualitatively, that members of the gray treefrog complex were common historically. Personal experience in the Southwest suggests that both species remain common. They occur densely around ponds in Alabama, but diffuse "populations" of calling Cope's gray treefrogs are found in suburban habitats.
Life History Features - Ritke et al. (1991a) discussed the life history of a western Tennessee population of Cope's Gray Treefrogs.
Breeding - Reproduction is aquatic.
Breeding migrations - Calling is probably stimulated by a combination of day length and temperature. Calling typically begins earlier in the southern portion of their range. Wright (1932) reports Dryophytes versicolor (almost certainly D. chrysoscelis) from the Okefenokee Swamp calling from 10 June–13 August. In northeastern Alabama, calling can begin in late March and continue through July, but calling is most intense in April–May. In north-central Oklahoma, calling usually begins in April, with a sharp peak in May to early June. Calling usually does not extend very far into July in Oklahoma. Fellers (1997b) reports calling activity in Maryland from April–July or August.
Cope's Gray Treefrogs breed from mid March to July (Wright and Wright, 1949). It seems reasonable to suggest that the peak of the breeding season for both species is late spring (May–June).
Breeding Habitat - During the breeding season, Cope's Gray Treefrogs are found calling near the edges of ponds, ephemeral wetlands, and ditches, and from floating algae and emergent vegetation (Fellers, 1979b; Godwin and Roble, 1983; Conant and Collins, 1998; personal observations). At dusk, Cope's Gray Treefrogs begin calling from high in the trees surrounding a pond. As the evening progresses, individuals move down the trees (sometimes calling along the way) until they reach lower branches or shrubs, or they continue until they reach the ground and move to a point usually within 1.5 m of the water’s edge (personal observations). Fellers (1979b) detailed calling site characteristics. Ptacek (1992) compared calling sites of D. chrysoscelis and D. versicolor. Godwin and Roble (1983) suggest that females may mate on the first day they arrive at the breeding pond. Males and females may mate ≤ 3 times/breeding season (Godwin and Roble, 1983). Ritke and Lessman (1994) present biopsy data indicating that females develop follicles throughout the breeding season, and production of later follicles relate to foraging success. Ritke et al. (1991b) report strong breeding pond philopatry.

Egg deposition sites - Egg deposition sites are similar for both Cope's Gray Treefrogs and Gray Treefrogs. Eggs are loosely attached to emergent vegetation at the surface of shallow ponds and pools (permanent or temporary). These wetlands may be natural or created and can be highly disturbed. Hillis et al. (1987) reports Cope's Gray Treefrogs breeding in the rain-filled furrows of cornfields in Kansas.
Clutch size - Eggs are laid in packets (10 x 12.5 cm, 30–40 eggs/packet) as a surface film loosely attached to emergent vegetation (Wright, 1932).
Altig & McDiarmid 2015 - Classification and Description:
- Eastern Film
- Arrangement 2 - Eggs oviposited as adherent films, individual eggs not easily removed from film, top of outer jelly lies at water surface; jelly diameter not large relative to ovum size. Film diameter less than 150 mm, ova pale to dark brown.
- Sub-arrangement B - Eggs deposited in ephemeral nonflowing water; Ovum Diameter 0.8-1.2 mm; 2 jelly layers.
- Arrangement 2 - Eggs oviposited as adherent films, individual eggs not easily removed from film, top of outer jelly lies at water surface; jelly diameter not large relative to ovum size. Film diameter less than 150 mm, ova pale to dark brown.
Larvae/Metamorphosis - Development is aquatic. Thibaudeaux and Altig (1998) documented the ontogenic development of the oral apparatus of Cope's Gray Treefrogs.
Tadpoles:
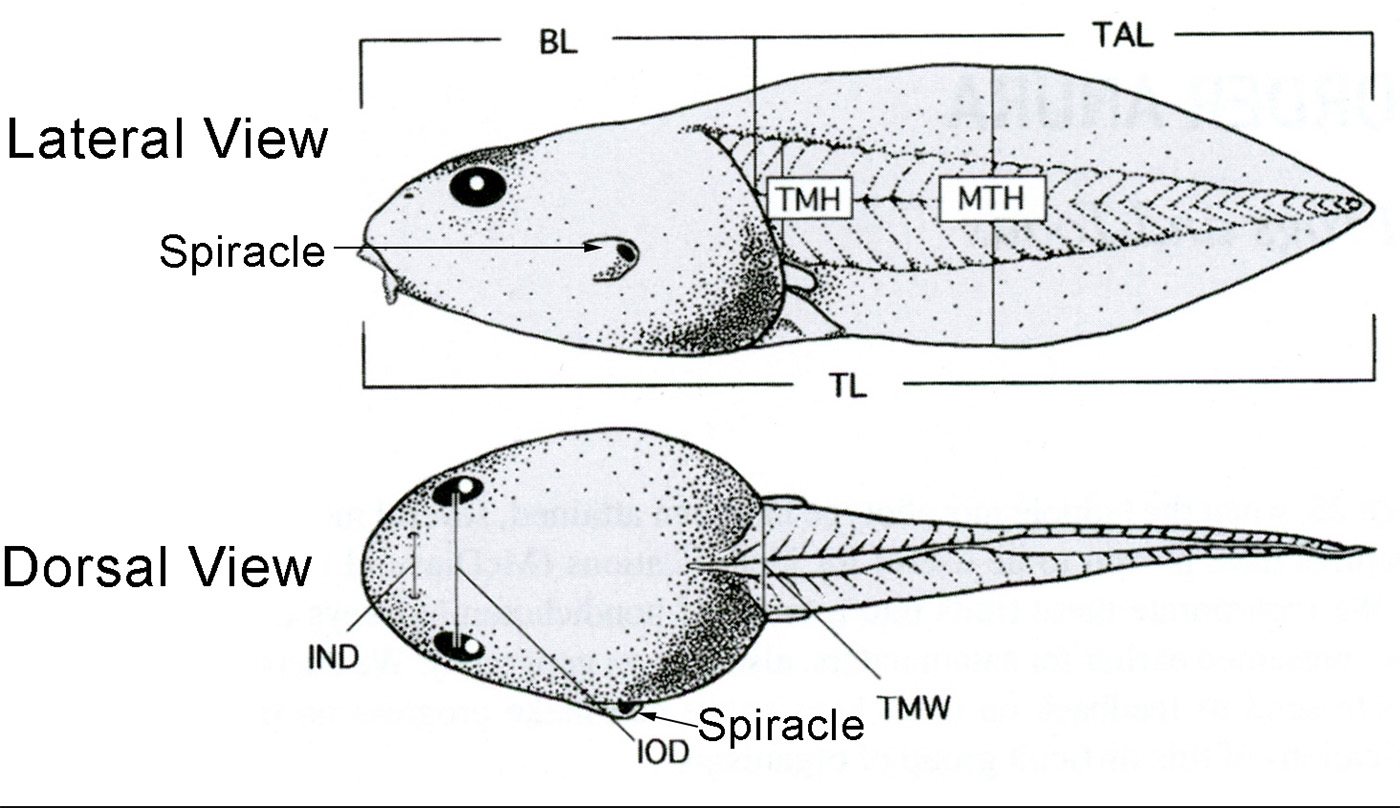
| Lateral View | Dorsal View |
|---|---|
| BL = Body Length | IND = Internarial Distance |
| MTH = Maximum Tail Height | IOD = Interorbital Distance |
| TAL = Tail Length | TMW = Tail Muscle Width |
| TL = Total Length | |
| TMH = Tail Muscle Height |
Length of larval stage - Dickerson (1906) reported a larval period of 3 wk from eggs to hatching and 4 wk to metamorphosis. Wright (1932) reported 45–65 d from eggs to metamorphosis. Because developmental rates are generally positively linked to temperature, one would expect that the average developmental times are shorter in the South.
Larval requirements - McDiarmid and Altig (1999) have summarized most of what has been published on tadpoles. The sources cited herein are largely from this text.
Larvae Food - No data are available on the food habits of Cope's Gray Treefrogs or Gray Treefrogs. Tadpoles generally feed by filtering food from the water column or by scraping periphyton from submerged substrates (Hoff et al., 1999). Steinwascher and Travis (1983) reported that Cope's Gray treefrog tadpoles grew faster on diets with high protein to carbohydrate ratios.
Cover - There are no published reports of cover requirements for Cope's Gray treefrog tadpoles. During the day, tadpoles can be seen resting on a variety of substrates including exposed sediment, leaf litter, and fallen tree limbs. I have observed hylid tadpoles resting on top of these same substrates at night.
Larval polymorphisms - McCollum and Van Buskirk (1996) reported that reddish and yellowish tail pigments in Cope's Gray Treefrogs were induced by the presence of odonate naiad predators.
Features of metamorphosis - Larval Cope's Gray Treefrogs are approximately 16 mm TL at metamorphosis (Wright, 1932).
Post-metamorphic migrations - Roble (1979) describes post-metamorphic migrations of Gray Treefrogs from central Wisconsin. Within a week, juveniles dispersed from their natal ponds. Juveniles moved an average of 1.58 m/d, with maximum dispersal distances approaching 125 m (Roble, 1979). Juveniles were active throughout the day from July–September.
Juvenile Habitat - Roble (1979) reported that juvenile Gray Treefrogs from Wisconsin were captured on sedges (Carex sp.) about 1/3 of the time, with false nettle (Boehmeria cylindrica), reed grass (Phalaris arundinacea), and swamp white oak (Quercus bicolor) saplings as preferred habitat. Nearly 3/4 (70.3%) of all captures were below 50 cm above the forest floor, while > 2% were found above 1.2 m. Roble (1979) surmised that young frogs did not ascend into trees during their first season.
Adult Habitat - Outside of the breeding season, Cope's Gray Treefrogs are found on trees or on mossy or lichen-covered fences, usually above ground (Conant and Collins, 1998), and will utilize knothole cavities (Ritke and Babb, 1991) and bluebird nesting boxes (personal observations).
Home Range Size - Little information is known regarding movements outside the breeding season. Adults are thought to spend the remaining part of the activity season high in trees where they forage on insects and insect larvae. Short-term movements are probably limited, but during dry seasons, low relative humidity may drive treefrogs to seek out high relative humidity microhabitats.
Territories - Little is known about territoriality in these frogs. Gray Treefrogs are known to produce specialized calls (called "turkey roots" by Wright, 1932) when approached while calling. Fellers (1979a) describes territorial behavior in treefrogs. Wells and Taigen (1986) note that call duration increases in high density (mean distance between individuals ~1 m) populations, presumably a response to intrusion in a territory.
Aestivation/Avoiding Dessication - Not documented.
Seasonal Migrations - The only migrations reported for gray treefrogs are those to the breeding ponds beginning in March and continuing through June. In July, individuals may call during periods of high humidity or after rains, but populations tend to be diffuse. Newly metamorphosed Gray Treefrogs move ≤ 1.1 km (0.7 mi) from the breeding ponds (Roble, 1979). Dispersal to winter hibernacula have not been described, but such movements are probably short and asynchronous.
Torpor (Hibernation) - Wright (1932) speculated that gray treefrogs remained active in Georgia "until November at least." Harlan (1835, in Wright, 1932) relates collecting a specimen several feet below the surface of the ground from under a root of an apple tree in winter. Burkholder (1998) discovered hibernacula of Cope's Gray Treefrogs near the bases of sugar maple trees (Acer saccharum), 2.5–5.0 cm below the soil surface, as well as within leaf litter of varying depths; all individuals were found < 1 m from the base of each tree investigated.
Freeze tolerance has been described for Gray Treefrogs. Schmid (1982) reported that glycerol production in Gray Treefrogs allowed individuals to survive –6 ˚C for 5–7 d. Storey and Storey (1985) reported additional production of glucose as a cryoprotectant. Gray treefrogs were able to survive –2 ˚C for 5 d and were also capable of surviving repeated freezing and thawing events. Storey and Storey (1986) found Gray Treefrogs could survive moderate freezing temperatures (–2 to –4 ˚C) for ≤ 2 wk. Cope's Gray Treefrogs exhibit natural freeze tolerance via production of elevated levels of glucose in their blood plasma (Costanzo et al., 1992).
Interspecific Associations/Exclusions - Maxson et al. (1977) reported immunological hybrids between D. chrysoscelis and D. versicolor in southeastern Oklahoma. Ralin et al. (1983) reported on electrophoretic hybrids from Illinois. Gerhardt et al. (1994) cytologically confirmed the natural occurrence of a triploid hybrid between Cope's Gray Treefrogs (diploid) and eastern gray treefrogs (tetraploid).
Petranka (1989b) reported possible chemical inhibition of southern leopard frog (R. sphenocephala) tadpoles by Cope's Gray treefrog tadpoles. Alford and Wilbur (1985) and Morin (1987) reported that Cope's Gray treefrog tadpoles were smaller in the presence of other tadpoles, and the order in which species appeared in a pond influenced the community composition at that pond.
Ralin (1981) demonstrated that Cope's Gray Treefrogs and Gray Treefrogs from Texas had similar abilities to cope with desiccation in the same habitats. He further noted greater variability among populations of the same species than he saw between the species.
Age/Size at Reproductive Maturity - Cope's Gray Treefrogs range in size from 32–60 mm (Wright and Wright, 1949; Conant and Collins, 1998). Wright (1932) suggests that Gray Treefrogs from the Okefenokee Swamp begin breeding at 2 yr of age.
Longevity - There are no published reports on longevity of Cope's Gray Treefrogs.
Feeding Behavior - General reports list insects as the prey of gray treefrogs (Holbrook, 1842, in Wright, 1932). Dickerson (1906) and Ritke and Babb (1991) found Gray Treefrogs to be "sit-and-wait" predators, consuming caterpillars, beetles, flies, wood roaches (Parcoblatta sp., Ischnoptera deropeltiformis), and camel crickets (Ceuthophilus sp.).
Predators - Numerous potential predators exist for hylids, ranging from invertebrates through vertebrates. Dickerson (1906) indicated that diving beetles preyed upon Cope's Gray treefrog tadpoles. Resetarits (1998) reported that odonate naiads and larval dytiscids (diving beetles) both preyed on Cope's Gray Treefrog tadpoles, but larval dytiscids were major egg predators as well. Dragonfly naiads were also used in experimental studies of tadpole response to predators (McCollum and Van Buskirk, 1996). A wide variety of fish could prey upon all life stages of Cope's Gray Treefrogs. Petranka et al. (1987) noted the ability of Cope's Gray Treefrog tadpoles to detect chemicals associated with fish predators (green sunfish, Lepomis cyanellus). Smith et al. (1999) reported that bluegill sunfish (Lepomis macrochirus) substantially reduced Gray Treefrog tadpole abundance in field experiments. Potential amphibian predators include salamanders and salamander larvae (chiefly newts [Notophthalmus sp.] and ambystomatids [Ambystoma sp.]) and some adult frogs (i.e., American Bullfrogs [Lithobates catesbeiana]). Skelly (1992) used tiger salamanders (Ambystoma tigrinum) in field experiments of antipredator costs. Numerous turtles and snakes represent potential predators on the various stages of the life cycle of frogs. Wading birds, especially herons (Ardeidae), prey upon tadpoles and frogs of many species. Additionally, raccoons (Procyon lotor) and skunks (Mephitis mephitis) are potential mammalian predators.
Anti-Predator Mechanisms - Cope's Gray Treefrogs produce mucous secretions that are foul tasting and cause burning sensation and inflammation in mucous membranes of eyes (personal observations). While these secretions have antipredator functions, it is possible that they also function as antimicrobial agents. Cline (1986) reported death feigning (thanatosis) in Cope's gray treefrogs from northeastern Oklahoma.
Tadpoles may not use chemical defense compounds. Kats et al. (1988) categorized Cope's gray treefrogs tadpoles as "palatable" in their predation studies. McCollum and Van Buskirk (1996) reported that predators (odonate naiads) induced production of reddish or yellowish tail pigments in Cope's Gray Treefrogs. Subsequently, two papers (Semlitsch, 1990; Figiel and Semlitsch, 1991) noted that substantial tail damage (> 75%) must occur before tadpoles suffer increased mortality (using odonate and crayfish predators). Thus, it appears that tadpoles distribute these pigments in the tail to misdirect predator attacks. Several studies have also reported decreased tadpole activity (Fauth, 1990) and a shift in habitat usage (Petranka et al., 1987; Kats et al., 1988) in the presence of predators. Resetarits and Wilbur (1989) concluded that Cope's Gray treefrog tadpoles were capable of detecting chemical odors of potential predators in water conditioned by these predators.
Diseases - So far, no known reports of hylid frog declines have been related to diseases such as those caused by chytrid fungi or ranaviruses.
Parasites - A variety of parasite hosts have been suggested for gray treefrogs. Armstrong et al. (1997) studied reproduction of a monogenean flatworm parasite in Cope's Gray Treefrogs. Delvinquier and Dresser (1996) reported an opalinid (Sarcomastigophora) from Gray Treefrogs. Hausfater et al. (1990) chose Gray Treefrogs as a model organism for studying the effect of parasitism on mate choice, in part because males harbored a “wide range of helminth parasites."
Conservation - Cope's Gray Treefrogs are moderately tolerant to the pollutants tested so far, and they are tolerant to human habitat disturbance.
Confusion between this species and Gray Treefrogs continues to confound conservation efforts. Cope's Gray Treefrogs are listed as Endangered in New Jersey, where a permit issued by the New Jersey Division of Fish, Game, and Wildlife is required for all activities involving this species.
References for Life History
- Altig, Ronald & McDiarmid, Roy W. 2015. Handbook of Larval Amphibians of the United States and Canada. Cornell University Press, Ithaca, NY. 341 pages.
- AmphibiaWeb. 2020. University of California, Berkeley, CA, USA.
- Conant, Roger and, Collins, John T., 2016, Peterson Field Guide: Reptiles and Amphibians, Eastern and Central North America, 494 pgs., Houghton Mifflin Company., New York
- Duellman, William E. and, Trueb, Linda, 1986, Biology of Amphibians, 671 pgs., The Johns Hopkins University Press, Baltimore
- Martof, B.S., Palmer, W.M., Bailey, J.R., Harrison, III J.R., 1980, Amphibians and Reptiles of the Carolinas and Virginia, 264 pgs., UNC Press, Chapel Hill, NC
- Wilson, L.A., 1995, Land manager's guide to the amphibians and reptiles of the South, 360 pp. pgs., The Nature Conservancy, Southeastern Region, Chapel Hill, NC
Photos:
*Click on a thumbnail for a larger version.
Verified County/City Occurrence
Accomack
Albemarle
Amelia
Appomattox
Arlington
Augusta
Bedford
Brunswick
Buchanan
Buckingham
Caroline
Carroll
Charles City
Chesterfield
Culpeper
Cumberland
Dickenson
Dinwiddie
Essex
Fairfax
Fauquier
Floyd
Fluvanna
Franklin
Frederick
Gloucester
Goochland
Greensville
Halifax
Hanover
Henrico
Henry
Isle of Wight
James City
King and Queen
King George
King William
Lancaster
Lee
Loudoun
Louisa
Lunenburg
Mathews
Mecklenburg
Middlesex
Montgomery
New Kent
Northampton
Northumberland
Nottoway
Orange
Patrick
Pittsylvania
Powhatan
Prince Edward
Prince George
Prince William
Richmond
Roanoke
Russell
Scott
Smyth
Southampton
Spotsylvania
Stafford
Surry
Sussex
Washington
Westmoreland
Wise
York
CITIES
Chesapeake
Hampton
Newport News
Norfolk
Suffolk
Virginia Beach
Williamsburg
Verified in 71 counties and 7 cities.
U.S. Range